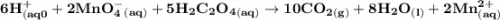Chemistry, 26.10.2020 16:40 Hannahdavy5434
A student dissolved a 0.139g sample of oxalic acid, H2C2O4, in water in an Erlenmeyer flask. Then the student titrated the H2C2O4 solution in the flask with a solution of KMnO4, which has a dark purple color. The balanced chemical equation for the reaction that occurred during the titration is shown above. (a) Identify the species that was reduced in the titration reaction. Justify your answer in terms of oxidation numbers.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
A student dissolved a 0.139g sample of oxalic acid, H2C2O4, in water in an Erlenmeyer flask. Then th...
Questions

Mathematics, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30

History, 15.07.2019 21:30

Chemistry, 15.07.2019 21:30

History, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30

History, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30


Mathematics, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30


Biology, 15.07.2019 21:30


Mathematics, 15.07.2019 21:30

English, 15.07.2019 21:30

History, 15.07.2019 21:30

Mathematics, 15.07.2019 21:30