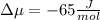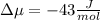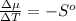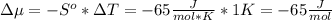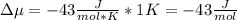Chemistry, 26.10.2020 16:40 corey36dylon
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the same temperature is 43 J K−1 mol−1. Calculate the change in chemical potential of liquid water and of ice when the temperature is increased by 1 K from the normal melting point. Giving your reasons, explain which phase is thermodynamically the more stable at the new temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the sam...
Questions

Mathematics, 15.01.2021 03:50




Social Studies, 15.01.2021 03:50


Mathematics, 15.01.2021 03:50

Social Studies, 15.01.2021 03:50

Mathematics, 15.01.2021 03:50


Mathematics, 15.01.2021 03:50






History, 15.01.2021 03:50

Mathematics, 15.01.2021 03:50

Biology, 15.01.2021 03:50

Mathematics, 15.01.2021 03:50