
Chemistry, 26.10.2020 16:40 jsmith4184
What molar ratio of acetic acid to sodium acetate is required to create a buffer solution having a pH of 4.93 at 25°C? Ka for HC2H3O2 is 1.8 × 10–5.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2


Chemistry, 23.06.2019 13:30
32p and 31p are two isotopes of phosphorus. compare the number if subatomic particles that are present in the atoms of these isotopes.
Answers: 1
You know the right answer?
What molar ratio of acetic acid to sodium acetate is required to create a buffer solution having a p...
Questions



Geography, 16.11.2019 03:31

Mathematics, 16.11.2019 03:31



English, 16.11.2019 03:31


Social Studies, 16.11.2019 03:31



Mathematics, 16.11.2019 03:31

Computers and Technology, 16.11.2019 03:31






Health, 16.11.2019 03:31

Mathematics, 16.11.2019 03:31

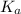 = Acid ionization constant of acetic acid =
= Acid ionization constant of acetic acid = 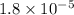
 = Potential of hydrogen of buffer solution =
= Potential of hydrogen of buffer solution = 
![K_a=\dfrac{[H+][A-]}{[HA]}\\\Rightarrow K_a=[H+]\dfrac{[A-]}{[HA]}](/tpl/images/0840/1638/61c39.png)
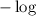 on both sides of the equation we get
on both sides of the equation we get![-\log K_a=-\log [H+]-\log\dfrac{[A-]}{[HA]}\\\Rightarrow pK_a=pH-\log\dfrac{[A-]}{[HA]}\\\Rightarrow pH=pK_a+\log\dfrac{[A-]}{[HA]}](/tpl/images/0840/1638/8d914.png)
![4.93=-\log(1.8\times 10^{-5})+\log\dfrac{[A-]}{[HA]}\\\Rightarrow 4.93-4.74=\log\dfrac{[A-]}{[HA]}\\\Rightarrow 0.19=\log\dfrac{[A-]}{[HA]}\\\Rightarrow 1.55=\dfrac{[A-]}{[HA]}\\\Rightarrow \dfrac{[HA]}{[A-]}=0.65](/tpl/images/0840/1638/a0e07.png)


