
Chemistry, 26.10.2020 17:20 justinchou814
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H is 1.00783 amuamu, and the mass of a neutron is 1.00866 amuamu .)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

You know the right answer?
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H i...
Questions

History, 06.10.2021 22:30

English, 06.10.2021 22:30

Computers and Technology, 06.10.2021 22:30


Biology, 06.10.2021 22:30


Computers and Technology, 06.10.2021 22:30

History, 06.10.2021 22:30


Mathematics, 06.10.2021 22:40



Mathematics, 06.10.2021 22:40

Mathematics, 06.10.2021 22:40


Mathematics, 06.10.2021 22:40


English, 06.10.2021 22:40

Mathematics, 06.10.2021 22:40

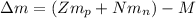
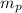 : is the proton mass = 1.00783 amu
: is the proton mass = 1.00783 amu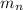 : is the neutron mass = 1.00866 amu
: is the neutron mass = 1.00866 amu ![\Delta m = (Zm_{p} + Nm_{n}) - M = [92*1.00783 amu + (239 - 92)*1.00866 amu] - 239.05429 amu = 1.93909 amu](/tpl/images/0840/4283/ccb79.png)



