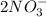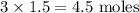When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent that process by the following equation: Ca(NO3)2(s) → Ca2+(aq) + 2NO3-(aq) When 1.5 mol calcium nitrate dissolves in water, how many moles of ions should be present in solution? Group of answer choices

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1
You know the right answer?
When calcium nitrate, Ca(NO3)2, dissolves in water, it dissociates into its ions. We can represent t...
Questions





Health, 20.07.2019 17:10

Mathematics, 20.07.2019 17:10


Advanced Placement (AP), 20.07.2019 17:10


Mathematics, 20.07.2019 17:10

English, 20.07.2019 17:10








Chemistry, 20.07.2019 17:10

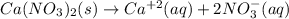
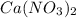 produces 2 moles of
produces 2 moles of 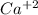 and 1 mole of
and 1 mole of