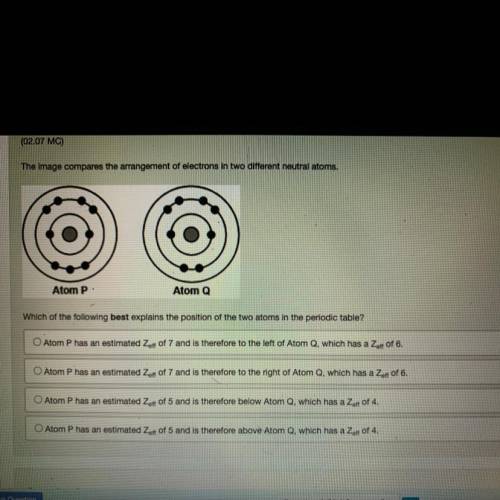
The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom...

Chemistry, 26.10.2020 19:40 Buttercream16
The image compares the arrangement of electrons in two different neutral atoms.
Atom P:
Atom Q
Which of the following best explains the position of the two atoms in the periodic table?
O Atom P has an estimated Zaff of 7 and is therefore to the left of Atom Q, which has a Zert of 6.
O Atom P has an estimated Zeft of 7 and is therefore to the right of Atom Q, which has a Zer of 6.
O Atom P has an estimated Zert of 5 and is therefore below Atom Q, which has a Zoff of 4.
O Atom P has an estimated Zoff of 5 and is therefore above Atom Q, which has a Zet of 4.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Questions

Social Studies, 01.08.2019 11:30




Social Studies, 01.08.2019 11:30

History, 01.08.2019 11:30

Mathematics, 01.08.2019 11:30

Mathematics, 01.08.2019 11:30

Mathematics, 01.08.2019 11:30

History, 01.08.2019 11:30

English, 01.08.2019 11:30




Social Studies, 01.08.2019 11:30

Health, 01.08.2019 11:30



Business, 01.08.2019 11:30



