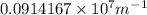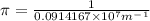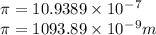Chemistry, 26.10.2020 22:50 oliviaprejean18
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls from the 6th Bohr orbit to the 3rd Bohr orbit.
a) 540 nm
b) 2000 nm
c) 1090 nm
d) 1050 nm

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Calculate the wavelength of light (in nm) of the spectral line of Hydrogen where an electron falls f...
Questions

Mathematics, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01

Physics, 16.10.2020 21:01

Chemistry, 16.10.2020 21:01


Social Studies, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

History, 16.10.2020 21:01

English, 16.10.2020 21:01


Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01

Spanish, 16.10.2020 21:01

Mathematics, 16.10.2020 21:01



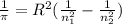
![[n_2n_1]](/tpl/images/0841/8494/b45f5.png)
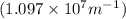
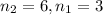
 wavelength of light
wavelength of light 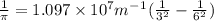
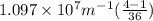
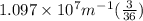
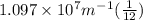
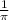 =
=