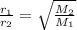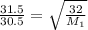Chemistry, 27.10.2020 04:30 andrew5632
16. The diffusion rate of an unknown gas is measured to be 31.50 mL/min. Under
identical conditions, the diffusion rate of oxygen gas is measured to be 30.50
mL/min. Hint: diffusion rate is directly related to velocity.
Determine the identity of the unknown gas from the following options:
a. CH4
b. CO
C. NO
d. CO2
e. NO2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 01:30
Ascientist is measuring the pressure that is exerted by each of the following gases in the atmosphere: carbon dioxide, oxygen, and nitrogen. which term most likely describes what she is measuring?
Answers: 1
You know the right answer?
16. The diffusion rate of an unknown gas is measured to be 31.50 mL/min. Under
identical conditions...
Questions



Mathematics, 11.02.2021 03:50

English, 11.02.2021 03:50

History, 11.02.2021 03:50


Mathematics, 11.02.2021 03:50

Mathematics, 11.02.2021 03:50


History, 11.02.2021 03:50

Mathematics, 11.02.2021 03:50





Mathematics, 11.02.2021 03:50

Mathematics, 11.02.2021 03:50

English, 11.02.2021 03:50

History, 11.02.2021 03:50

Biology, 11.02.2021 03:50