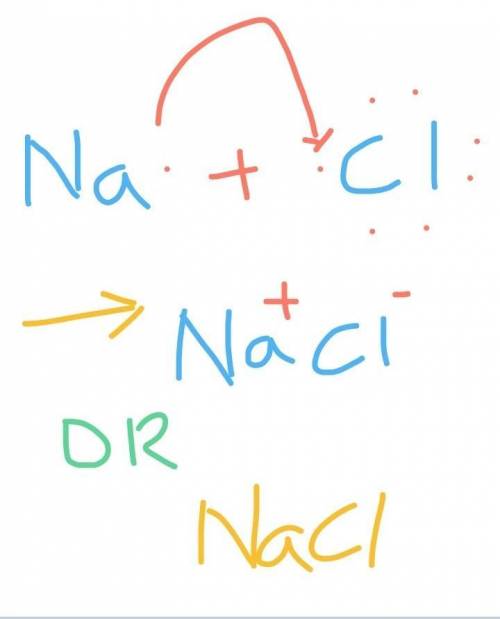
Chemistry, 27.10.2020 17:10 twrxght6643
How valence electron of sodium(Na) and react with valence electrons of Chlorine (CI)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
How valence electron of sodium(Na) and react with valence electrons of Chlorine (CI)...
Questions



Chemistry, 17.02.2021 19:30

Engineering, 17.02.2021 19:30



Spanish, 17.02.2021 19:30

Biology, 17.02.2021 19:30







Mathematics, 17.02.2021 19:30


Mathematics, 17.02.2021 19:30



Mathematics, 17.02.2021 19:30