
Chemistry, 27.10.2020 17:20 mvongphakdy8124
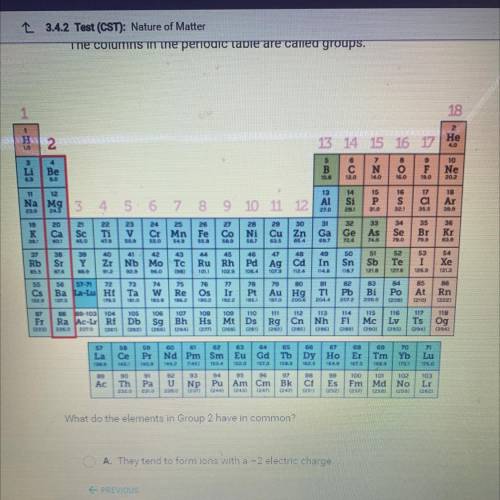
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge.
B. They tend to form ions with a +2 electric charge.
C. They are all highly reactive nonmetals.
D. They are all found in nature in their pure forms.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 11:30
Place the following substances in order of ph from lowest ph to highest. a. neutral compounds, bases, acids b. acids, neutral compounds, bases c. bases, acids, neutral compounds d. bases, neutral compounds, acids
Answers: 1
You know the right answer?
What do the elements in Group 2 have in common?
A. They tend to form ions with a -2 electric charge...
Questions





Advanced Placement (AP), 06.12.2020 01:40



Health, 06.12.2020 01:40

Physics, 06.12.2020 01:50


History, 06.12.2020 01:50



Physics, 06.12.2020 01:50

English, 06.12.2020 01:50

History, 06.12.2020 01:50

English, 06.12.2020 01:50

Geography, 06.12.2020 01:50


Engineering, 06.12.2020 01:50



