
Chemistry, 15.11.2019 20:31 JessTaylr04
1. nuclear decay by alpha particle emission is more common in atoms of elements that:
a have an atomic number greater than 83
b have a proton to neutron ratio of greater than 3: 1
c have an atomic number less than 83
d have more electrons than protons
2. identify the missing particle in the following equation: 3/2he + 3/2he -> 2 1/1he +
(3/2he refers to 3 above 2 next to he as an isotope symbol)
a 2/1he
b 1/1he
c 5/3li
d 4/2he
3. one of the major drawbacks to using nuclear fission as a reliable energy source is:
a there is not enough energy produced by this method to make it profitable.
b the process cannot be well-controlled.
c the intense amount of heat require to initiate the reaction.
d the products are more radioactive than the reactants.
4. which of the following concerns apply to nuclear fission reactions?
a concern about the disposal of nuclear waste
b concern about the safe operation of nuclear plants
c concern about nuclear weapons proliferation
d all of these are concerns
5. the energy output of the sun and other stars is a result of fission reactions among hydrogen nuclei.
true
false
6. why is the total mass of a helium nucleus not equal to the mass of its individual parts? (points : 3)
a the "missing" mass is due to inaccurate measurement on a very small scale.
b the "missing" mass has been converted to energy.
c the "missing" matter has been converted to mass.
d the "missing" mass has been converted to matter.
7. in a nuclear reaction, the mass-energy of the reactants is generally: (points : 3)
a less than the mass-energy of the products
b equal to the mass of the products
c equal to the energy of the products
d equal to the mass-energy of the products
8. a mass of 10.0 kilograms is completely converted into energy. what is this amount of energy in joules? (1 j = 1 kg m2/s2) (points : 3)
a 3.33 x 10^8 j
b 1.11 x 10^-8 j
c 3.00 x 10^16 j
d 9.00 x 10^17
9. a mass of 4.00 kilograms is completely converted into energy. what is this amount of energy in joules? (1 j = 1 kg m2/s2) (points : 3)
a 1.20 x 10^9 j
b 1.33 x 10^-8 j
c 3.60 x 10^17 j
d 4.4 x 10^-16 j
10. according to einstein's formula for the conversion of mass and energy, to find the energy, multiply the mass by the speed of light. (points : 2)
true
false

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
1. nuclear decay by alpha particle emission is more common in atoms of elements that:
a have...
a have...
Questions




English, 27.05.2020 09:59


Computers and Technology, 27.05.2020 09:59

Mathematics, 27.05.2020 09:59


History, 27.05.2020 09:59


Mathematics, 27.05.2020 09:59


Mathematics, 27.05.2020 10:57

Mathematics, 27.05.2020 10:57

History, 27.05.2020 10:57

Mathematics, 27.05.2020 10:57

Mathematics, 27.05.2020 10:57



Mathematics, 27.05.2020 10:57

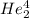
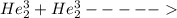
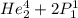
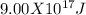
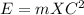 formula can be used.
formula can be used.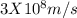
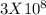 =
= 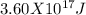 .
.

