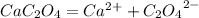Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2


Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Calcium oxalate (cac2o4) has a ksp value of 2.3 × 10–9 at 25°c. calculate the molar solubility of ca...
Questions


Biology, 27.08.2019 16:40


Business, 27.08.2019 16:40

History, 27.08.2019 16:40

History, 27.08.2019 16:40


Mathematics, 27.08.2019 16:40

Mathematics, 27.08.2019 16:40



History, 27.08.2019 16:40

Mathematics, 27.08.2019 16:40

English, 27.08.2019 16:40

Mathematics, 27.08.2019 16:40