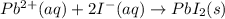Chemistry, 17.09.2019 04:30 kyliegriffis
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
which ion(s) would not be spectator ions?
a) pb2+ , no3-
b) k+ , i
c) no3- , pb2+
d) pb2+, i –
e) all the above ions are in the net ionic equation

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
Considering the following precipitation reaction: pb(no3)2(aq) + 2ki(aq) → pbi2(s) + 2kno3(aq),
Questions

Mathematics, 05.09.2020 19:01






Mathematics, 05.09.2020 19:01

Mathematics, 05.09.2020 19:01

History, 05.09.2020 19:01



Mathematics, 05.09.2020 19:01

Mathematics, 05.09.2020 19:01

Advanced Placement (AP), 05.09.2020 19:01




Mathematics, 05.09.2020 19:01


Mathematics, 05.09.2020 19:01

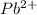 and
and 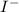
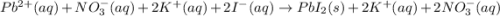
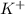 and
and 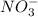 and are spectator ions.
and are spectator ions.