
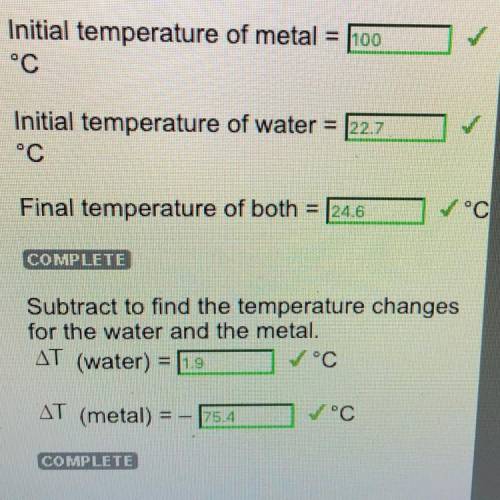
Step 7: Put the Metal in the water and Measure Temperature Changes (Copper) Initial temperature of metal
= 100°c
Initial temperature of water = 22.7°C
Final temperature of both
24.6°C
Subtract to find the temperature changes
for the water and the metal.
AT (water) = 1.9
AT (metal) = -75.4

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3


Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3
You know the right answer?
Step 7: Put the Metal in the water and Measure Temperature Changes (Copper) Initial temperature of m...
Questions


Mathematics, 13.03.2022 09:30




English, 13.03.2022 09:30

Mathematics, 13.03.2022 09:30


Mathematics, 13.03.2022 09:30

History, 13.03.2022 09:30



Mathematics, 13.03.2022 09:30

Mathematics, 13.03.2022 09:30


Mathematics, 13.03.2022 09:30

Mathematics, 13.03.2022 09:30






