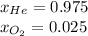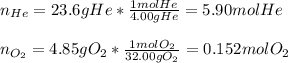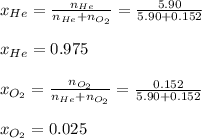Chemistry, 28.10.2020 16:30 dontcareanyonemo
A 13.0-L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 23.6 g of He and 4.85 g of O2 at 298 K. Calculate the mole fraction of each component in the mixture.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3
You know the right answer?
A 13.0-L scuba diving tank contains a helium-oxygen (heliox) mixture made up of 23.6 g of He and 4.8...
Questions

Mathematics, 12.03.2021 01:00


English, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00


Mathematics, 12.03.2021 01:00

Arts, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00



Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00

Mathematics, 12.03.2021 01:00