Chemistry, 28.10.2020 16:50 jholland03
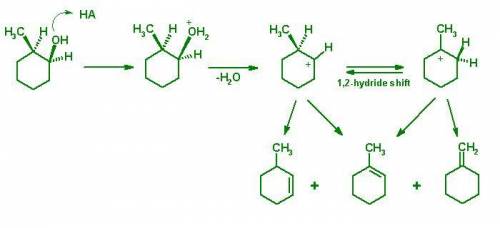
The 2-methylcyclohexanol used in this experiment is a mixture of cis and trans isomers, approximately in 1:1 ratio. Which kind of mechanism can better account for the product mixture obtained from the dehydration of cis and trans-2-methylcyclohexanol mixture: E1, E2, or a combination of the two

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
The 2-methylcyclohexanol used in this experiment is a mixture of cis and trans isomers, approximatel...
Questions


Mathematics, 21.02.2020 07:55

Mathematics, 21.02.2020 07:55

Mathematics, 21.02.2020 07:55

Mathematics, 21.02.2020 07:55

Chemistry, 21.02.2020 07:55

Spanish, 21.02.2020 07:56

Mathematics, 21.02.2020 07:56


Computers and Technology, 21.02.2020 07:56



Mathematics, 21.02.2020 07:57

Social Studies, 21.02.2020 07:57

Mathematics, 21.02.2020 07:57



Mathematics, 21.02.2020 07:58