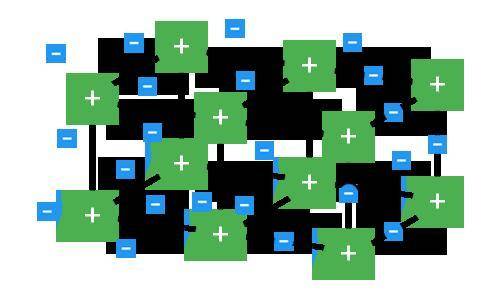
Which type of compound is represented in the model and why?
Select all that apply.
A. T...

Which type of compound is represented in the model and why?
Select all that apply.
A. This model is not a metallic compound because the electrons are being transferred between different ions in the crystal structure.
B. This model is an ionic compound because the rigid formation is an example of a crystal lattice.
C. This model is a metallic compound because it consists of free-moving electrons in a crystal formation.
D. This model is not an ionic compound because the electrons are not being transferred between positive and negative ions; instead, only positive ions are present.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

You know the right answer?
Questions



Social Studies, 25.08.2019 23:00





English, 25.08.2019 23:00




Biology, 25.08.2019 23:00

Mathematics, 25.08.2019 23:00

Social Studies, 25.08.2019 23:00

History, 25.08.2019 23:00

English, 25.08.2019 23:00


Business, 25.08.2019 23:00




