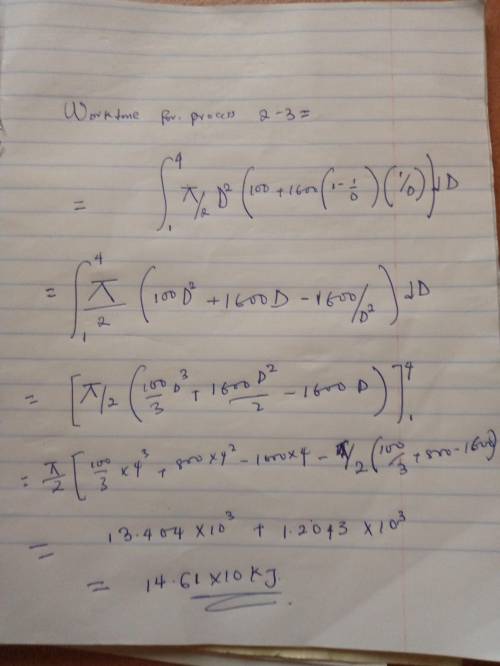Chemistry, 28.10.2020 16:40 adambbogard1589
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium gas at 1 MPa at ambient temperature of 20 degrees C. The valve is open and the balloon is inflated at constant pressure of 100 kPa (atmospheric pressure) until it becomes spherical at D1 = 1m. If the balloon is larger than this, the balloon material is stretched giving a pressure inside as:
P = Po + C(1-(D1/D))(D1/D)
The balloon is slowly inflated to a final diameter of 4m, at which point the pressure inside is 400 kPa. The temperature remains constant at 20 degrees C. Determine the work done during the overall process.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

You know the right answer?
An initially deflated and flat balloon is connected by a valve to a storage tank containing helium g...
Questions

Mathematics, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

Chemistry, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

Computers and Technology, 02.11.2020 17:20



History, 02.11.2020 17:20




Geography, 02.11.2020 17:20

Mathematics, 02.11.2020 17:20

Health, 02.11.2020 17:20





Mathematics, 02.11.2020 17:20