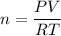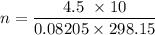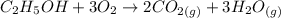Chemistry, 28.10.2020 16:40 brayannnnn36781
A 22.5 mL sample of liquid ethanol (C₂H₅OH, density = 0.789 g/mL) was injected into a 10.0 L cylinder containing O₂ at a pressure of 4.50 atm and a temperature of 25°C. The cylinder was heated to 125°C and a spark was used to ignite the ethanol, which was completely combusted. What was the final total pressure in the cylinder (in atm) after the reaction, while it was still at 125°C?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
A 22.5 mL sample of liquid ethanol (C₂H₅OH, density = 0.789 g/mL) was injected into a 10.0 L cylinde...
Questions


Mathematics, 16.10.2020 09:01

English, 16.10.2020 09:01




English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01




Mathematics, 16.10.2020 09:01

English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01

History, 16.10.2020 09:01


Biology, 16.10.2020 09:01



Mathematics, 16.10.2020 09:01