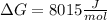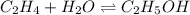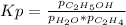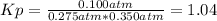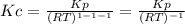Chemistry, 28.10.2020 17:10 Anasiabrown11
g what is ΔG for the reaction at 298K, when the partial pressure of C2H4 is 0.275 atm, the partial pressure of H2O is 0.350 atm, and the partial pressure of C2H5OH is 0.100 atm?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
g what is ΔG for the reaction at 298K, when the partial pressure of C2H4 is 0.275 atm, the partial...
Questions




Mathematics, 18.05.2021 15:40

Mathematics, 18.05.2021 15:40



Mathematics, 18.05.2021 15:40

Mathematics, 18.05.2021 15:40

Chemistry, 18.05.2021 15:40


Social Studies, 18.05.2021 15:40



Mathematics, 18.05.2021 15:40

English, 18.05.2021 15:40

German, 18.05.2021 15:40


English, 18.05.2021 15:40