PLEASE HELP ASAP
Step 1
Using the sticky notes, label the thermometers T1 and T2. Make s...

Chemistry, 29.10.2020 06:10 nicolesan3
PLEASE HELP ASAP
Step 1
Using the sticky notes, label the thermometers T1 and T2. Make sure that both thermometers are at room temperature (around 21°C). Then, in the table, record their temperatures and the time of this initial measurement.
Step 2
Place 1 tablespoon of baking soda in the small glass or jar. Carefully add one-fourth cup of white vinegar. When the mixture starts to bubble or fizz, place the first thermometer (T1) near (not in!) the glass. Then cover the glass and the thermometer with one of the upside-down soda bottles. If the thermometer cannot stand vertically on its own or it is too large to lay horizontally within the soda bottle, it can lean against an inner side of the soda bottle.
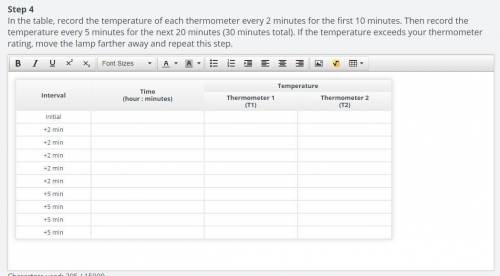
Step 3
Immediately place the other soda bottle upside down over the second thermometer (T2). Place each bottle approximately 4 to 5 inches apart under the lamp or other heat source. Turn on the lamp to expose each bottle to heat. The lamp or heat source represents the radiant energy that Earth receives from the Sun. The gases inside the bottles represents two different atmospheric compositions. Determine how the amount of radiant energy absorbed by each atmosphere changes by tracking the temperature in the table.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Questions


Spanish, 23.09.2019 04:00

Arts, 23.09.2019 04:00



History, 23.09.2019 04:00


Business, 23.09.2019 04:00


Biology, 23.09.2019 04:00

History, 23.09.2019 04:00

English, 23.09.2019 04:00


Social Studies, 23.09.2019 04:00




Biology, 23.09.2019 04:00

Mathematics, 23.09.2019 04:00



