
Chemistry, 29.10.2020 08:50 stormserena
a mixture of CaCO3 and CaO has a mass of 3.250g. After heating, the mass of the sample is reduced to 2.664g. What is the mass percent of CaCO3 in the mixture?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 06:00
In an exothermic reaction at equilibrium, what is the effect of lowering the temperature? a. the reaction makes more products. b. the reaction makes more reactants. c. the reaction is unchanged.
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2
You know the right answer?
a mixture of CaCO3 and CaO has a mass of 3.250g. After heating, the mass of the sample is reduced to...
Questions


Health, 23.09.2019 09:30

History, 23.09.2019 09:30

Chemistry, 23.09.2019 09:30

Physics, 23.09.2019 09:30



Geography, 23.09.2019 09:30

Mathematics, 23.09.2019 09:30

English, 23.09.2019 09:30

History, 23.09.2019 09:30



Social Studies, 23.09.2019 09:50


Mathematics, 23.09.2019 09:50




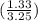 ×100%
×100%

