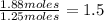Chemistry, 29.10.2020 17:00 lilinicholeb
An analysis shows that 2.97 g of iron metal combines with oxygen to form 4.25 g of an oxide of iron. What is the empirical formula of the compound

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1

Chemistry, 23.06.2019 07:20
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3
You know the right answer?
An analysis shows that 2.97 g of iron metal combines with oxygen to form 4.25 g of an oxide of iron....
Questions



Mathematics, 13.12.2021 16:50

English, 13.12.2021 16:50

Mathematics, 13.12.2021 16:50


Mathematics, 13.12.2021 16:50

Mathematics, 13.12.2021 16:50

Chemistry, 13.12.2021 16:50



Business, 13.12.2021 16:50

Social Studies, 13.12.2021 16:50

Health, 13.12.2021 16:50

Mathematics, 13.12.2021 16:50

Chemistry, 13.12.2021 16:50

Mathematics, 13.12.2021 16:50

English, 13.12.2021 16:50

Mathematics, 13.12.2021 16:50

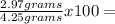 69.88%
Oxygen (O):
69.88%
Oxygen (O): 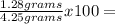 30.12%
30.12%
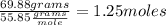 Oxygen (O):
Oxygen (O): 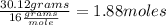
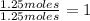 Oxygen (O):
Oxygen (O):