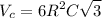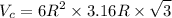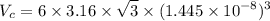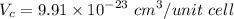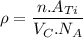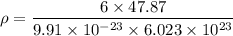Chemistry, 29.10.2020 17:10 estefaniapenalo
Titanium has an HCP unit cell for which the ratio of the lattice parameters c/a is 1.58. If the radius of the Ti atom is 0.1445 nm, calculate the density of Ti and compare it with the literature value of 4.51 g/cm3 . The atomic mass of titanium is 47.87 g/mol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

You know the right answer?
Titanium has an HCP unit cell for which the ratio of the lattice parameters c/a is 1.58. If the radi...
Questions



English, 31.12.2021 09:50

Medicine, 31.12.2021 09:50





Physics, 31.12.2021 09:50


Physics, 31.12.2021 09:50


Computers and Technology, 31.12.2021 09:50


Mathematics, 31.12.2021 09:50


Mathematics, 31.12.2021 09:50



Mathematics, 31.12.2021 14:00