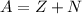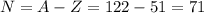Chemistry, 29.10.2020 19:20 mianelson367
An atom of antimony has a mass number of 122. The atomic number of antimony is 51. Calculate the number of protons and neutrons in this atom – work must be shown to earn credit.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
An atom of antimony has a mass number of 122. The atomic number of antimony is 51. Calculate the num...
Questions





Mathematics, 01.04.2020 16:11





Mathematics, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11



Chemistry, 01.04.2020 16:11

English, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11

Medicine, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11

Mathematics, 01.04.2020 16:11