
Chemistry, 30.10.2020 01:20 tnbankspines
I NEED HELP WITH ALL THESE QUESTIONS ILL GIVE BRAINLIEST AND THERES 19 POINTS! HELP ME PLZ

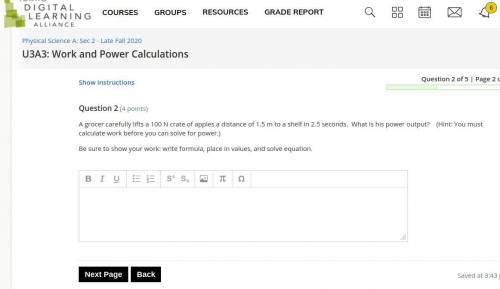
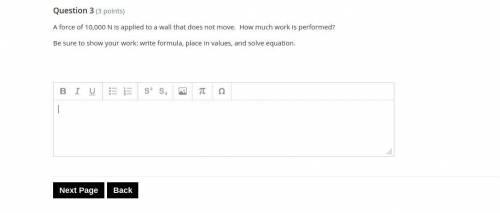
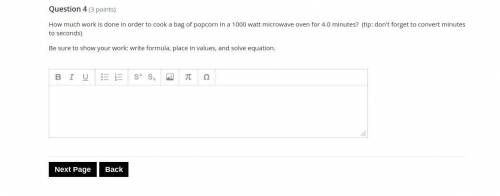
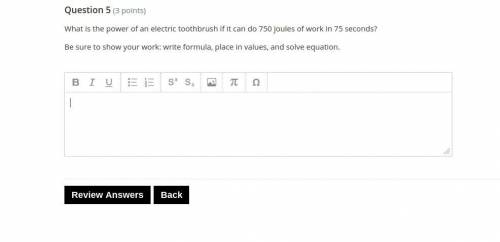

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1
You know the right answer?
I NEED HELP WITH ALL THESE QUESTIONS ILL GIVE BRAINLIEST AND THERES 19 POINTS! HELP ME PLZ
Questions

Mathematics, 29.01.2020 05:50


Biology, 29.01.2020 05:50

Geography, 29.01.2020 05:50


Biology, 29.01.2020 05:50

Physics, 29.01.2020 05:50



History, 29.01.2020 05:50

English, 29.01.2020 05:50





Mathematics, 29.01.2020 05:50


Computers and Technology, 29.01.2020 05:50





