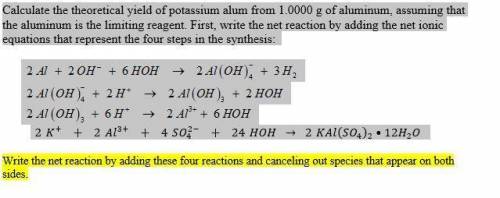
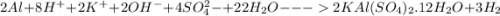Calculate the theoretical yield of potassium alum from 1.0000 g of aluminum, assuming that the aluminum is the limiting reagent. First, write the net reaction by adding the net ionic equations that represent the four steps in the synthesis:
(view image for reactions)
Write the net reaction by adding these four reactions and canceling out species that appear on both sides.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1


Chemistry, 23.06.2019 11:30
Jenny places a strip of ph paper into a solution. when she removes the ph paper, it has turned yellow-green. what should jenny do next to determine the ph of her solution? a. use a different testing method because the ph paper should not change colors b. place the ph paper into a machine that reads the ph of the solution c. compare the ph paper's color with the color of ph paper from another solution d. compare the ph paper's color with a chart of colors and ph ranges
Answers: 1

You know the right answer?
Calculate the theoretical yield of potassium alum from 1.0000 g of aluminum, assuming that the alumi...
Questions


Advanced Placement (AP), 30.11.2020 18:00











Chemistry, 30.11.2020 18:00

Mathematics, 30.11.2020 18:00

History, 30.11.2020 18:00




Mathematics, 30.11.2020 18:00

Spanish, 30.11.2020 18:00