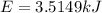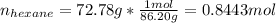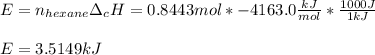The combustion of hexane is given by the following reaction. 2 C 6 H 14 + 19 O 2 → 12 CO 2 + 14 H 2 O The enthalpy of reaction is −4163.0 kJ/mol. How much energy (in joules) will be released if 72.78 grams of hexane is burned. (Molar mass of hexane = 86.20 g/mol).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
The combustion of hexane is given by the following reaction. 2 C 6 H 14 + 19 O 2 → 12 CO 2 + 14 H 2...
Questions

Mathematics, 23.04.2020 22:43

Mathematics, 23.04.2020 22:44



Mathematics, 23.04.2020 22:44

Engineering, 23.04.2020 22:44

English, 23.04.2020 22:44


Chemistry, 23.04.2020 22:44


Health, 23.04.2020 22:45




Social Studies, 23.04.2020 22:45


Mathematics, 23.04.2020 22:45

Mathematics, 23.04.2020 22:45

Computers and Technology, 23.04.2020 22:45

English, 23.04.2020 22:45