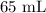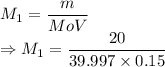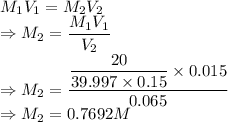Chemistry, 30.10.2020 16:50 kaelah6846
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of solution. She then took 15.0 mL of the stock solution and diluted it with enough water to make 65.0 mL of a final solution. What is the concentration of NaOH for the final solution?
A) O. 411 M
B) 0.534 M
C) 1.87 M
D) 2.43 M

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1


Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
A student prepared a stock solution by dissolving 20.0 g of NaOH in enough water to make 150. mL of...
Questions

Arts, 05.03.2021 20:50

Mathematics, 05.03.2021 20:50

Mathematics, 05.03.2021 20:50

Mathematics, 05.03.2021 20:50


Arts, 05.03.2021 20:50

Mathematics, 05.03.2021 20:50



Mathematics, 05.03.2021 20:50


Mathematics, 05.03.2021 20:50

English, 05.03.2021 20:50




Mathematics, 05.03.2021 20:50


Health, 05.03.2021 20:50

Mathematics, 05.03.2021 20:50

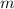 = Mass of sample =
= Mass of sample = 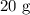
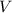 = Volume of solution =
= Volume of solution = 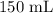
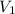 = Initial volume taken out of the stock solution =
= Initial volume taken out of the stock solution = 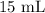
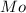 = Molar mass of NaOH =
= Molar mass of NaOH = 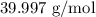
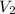 = Final volume of solution =
= Final volume of solution =