

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
A gaseous compound Y contains carbon and hydrogen only and has a density
of 1.696 g dm-3 at 95.3 kP...
Questions



Physics, 20.07.2019 10:00



Chemistry, 20.07.2019 10:00

History, 20.07.2019 10:00



History, 20.07.2019 10:00


Physics, 20.07.2019 10:00

Mathematics, 20.07.2019 10:00


Mathematics, 20.07.2019 10:00


Mathematics, 20.07.2019 10:00

Mathematics, 20.07.2019 10:00

Business, 20.07.2019 10:00

English, 20.07.2019 10:00

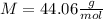
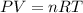
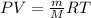
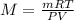
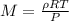
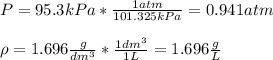
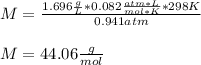
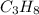 ) since it molar mass is 44.11 g/mol.
) since it molar mass is 44.11 g/mol.

