

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which is true of transition metals when moving from left to right on the periodic table? the d sublevels are not filled across the period. the cation radii become larger across the period. atomic radii increase slightly and then start to decrease. atomic radii decrease slightly and then start to increase. o
Answers: 2

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
Calculate the energy of the violet light emitted by a hydrogen atom with a wavelength of 410.1 nm....
Questions




Arts, 27.06.2019 21:30



Mathematics, 27.06.2019 21:30


English, 27.06.2019 21:30

Advanced Placement (AP), 27.06.2019 21:30

History, 27.06.2019 21:30

Mathematics, 27.06.2019 21:30






Mathematics, 27.06.2019 21:30

Mathematics, 27.06.2019 21:30

Chemistry, 27.06.2019 21:30

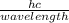
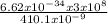 = 4.85 x 10⁻¹⁹J
= 4.85 x 10⁻¹⁹J

