
Chemistry, 02.11.2020 05:20 loveuncondition
Solid magnesium, Mg, reacts with oxygen gas, 02 to form the solid magnesium oxide, MgO. Which is a balanced chemical equation for this reaction?
A. Mg + MgO
B. Mg + O2 + MgO,
C. Mg + 20 2MgO
D. 2Mg +0,- 2MgO
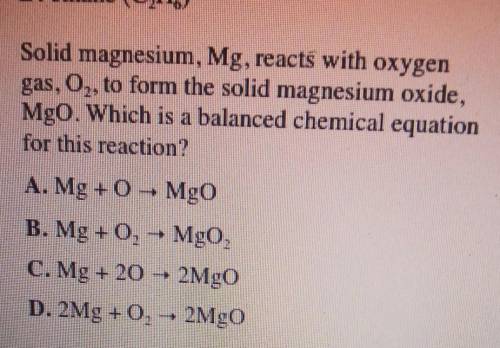

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Solid magnesium, Mg, reacts with oxygen gas, 02 to form the solid magnesium oxide, MgO. Which is a b...
Questions

Health, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01

Social Studies, 13.10.2020 16:01


Health, 13.10.2020 16:01



English, 13.10.2020 16:01

History, 13.10.2020 16:01




Mathematics, 13.10.2020 16:01

Medicine, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01


Mathematics, 13.10.2020 16:01



