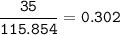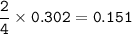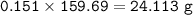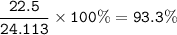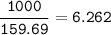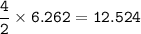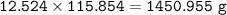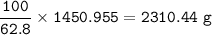Chemistry, 02.11.2020 06:30 Svetakotok
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What is the percentage yield of the reaction?
b) What mass of FeCO3 with a purity of 62.8% is needed to make 1.00 kg of Fe2O3?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
4 FeCO3 + O2 --> 2 Fe2O3 + 4CO2
a) A 35.0 g sample of pure FeCO3 produces 22.5 g of Fe2O3. What...
Questions



English, 22.08.2019 17:00

Mathematics, 22.08.2019 17:00

Social Studies, 22.08.2019 17:00

History, 22.08.2019 17:00


Mathematics, 22.08.2019 17:00

Health, 22.08.2019 17:00

Social Studies, 22.08.2019 17:00

Mathematics, 22.08.2019 17:00

Arts, 22.08.2019 17:00

Mathematics, 22.08.2019 17:00

Social Studies, 22.08.2019 17:00


Chemistry, 22.08.2019 17:00



Mathematics, 22.08.2019 17:00

Mathematics, 22.08.2019 17:00