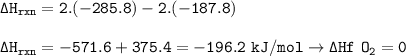Chemistry, 02.11.2020 14:00 steve12335
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combustion of a gram of hydrocar-
bon is between 40 and 50 kJ.
Practice Exercise 1
Calculate the enthalpy change for the reaction
2 H2O2(l)-→ 2 H2O(l) + O2(g)
using enthalpies of formation:
Change in enthalpy(f)H2O2(l)= -187.8 kJ/mol
Change in enthalpy (f) [H2O(l)= -285.8 kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
Comment Both propane and benzene are hydrocarbons. As a rule,
the energy obtained from the combusti...
Questions


Mathematics, 17.12.2020 14:00

Chemistry, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00

Mathematics, 17.12.2020 14:00



English, 17.12.2020 14:00


Mathematics, 17.12.2020 14:00

Arts, 17.12.2020 14:00

English, 17.12.2020 14:00

Arts, 17.12.2020 14:00




Social Studies, 17.12.2020 14:00


Mathematics, 17.12.2020 14:00