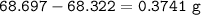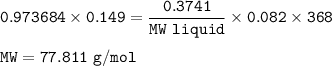Chemistry, 02.11.2020 14:10 joycewingate919
An empty 149 mL flask weighs 68.322 g before a sample of volatile liquid is added. The flask is then placed in a hot (95.0°C) water bath; the barometric pressure is 740 torr. The liquid vaporizes and the gas fills the flask. After cooling, flask and condensed liquid together weigh 68.697 g. What is the molar mass of the liquid?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
An empty 149 mL flask weighs 68.322 g before a sample of volatile liquid is added. The flask is then...
Questions

English, 06.11.2019 16:31






History, 06.11.2019 16:31


Social Studies, 06.11.2019 16:31

Mathematics, 06.11.2019 16:31

English, 06.11.2019 16:31

Physics, 06.11.2019 16:31


English, 06.11.2019 16:31




Business, 06.11.2019 16:31


Chemistry, 06.11.2019 16:31