

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? (HCl molar...
Questions

Chemistry, 20.10.2020 18:01


Mathematics, 20.10.2020 18:01





Biology, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01

Physics, 20.10.2020 18:01


Mathematics, 20.10.2020 18:01





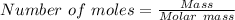
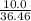
 mole of BCl₃ would be needed to produce 0.27427 mole of HCl
mole of BCl₃ would be needed to produce 0.27427 mole of HCl



