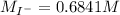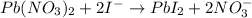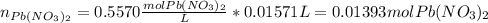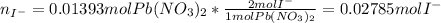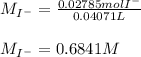Chemistry, 02.11.2020 16:30 KillerMDFK7992
The iodide ion concentration in a solution may be determined by the precipitation of lead iodide. Pb2 (aq) 2I-(aq) PbI2(s) A student finds that 15.71 mL of 0.5770 M lead nitrate is needed to precipitate all of the iodide ion in a 25.00-mL sample of an unknown. What is the molarity of the iodide ion in the student's unknown

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 15:30
Among these processes, which is the slowest chemical reaction? a. digesting food b. boiling an egg c. tarnishing of silver d. melting of a glacier
Answers: 2
You know the right answer?
The iodide ion concentration in a solution may be determined by the precipitation of lead iodide. Pb...
Questions











Mathematics, 11.11.2020 17:50


Mathematics, 11.11.2020 17:50