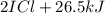Chemistry, 02.11.2020 16:40 guzmangisselle
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g) that reacts.
Write a balanced thermochemical equation for the reaction with an energy term in kJ as part of the equation. Note that the answer box for the energy term is case sensitive.
Use the SMALLEST INTEGER coefficients possible and put the energy term (including the units) in the last box on the appropriate side of the equation. If a box is not needed, leave it blank.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
When I2(g) reacts with Cl2(g) to form ICl(g) , 26.8 kJ of energy are evolved for each mole of I2(g)...
Questions








Spanish, 09.07.2019 20:00



English, 09.07.2019 20:00

Social Studies, 09.07.2019 20:00

Mathematics, 09.07.2019 20:00


History, 09.07.2019 20:00


English, 09.07.2019 20:00


Mathematics, 09.07.2019 20:00

History, 09.07.2019 20:00

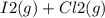 →
→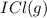
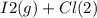 →
→ 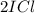
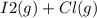 →
→