
Chemistry, 02.11.2020 21:00 SoccerdudeDylan
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water so that the final volume of the solution is 2.50 L . Calculate the molarity of the MgCl2 solution.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
An aqueous magnesium chloride solution is made by dissolving 7.15 moles of MgCl2 in sufficient water...
Questions

History, 11.10.2020 09:01

Health, 11.10.2020 09:01

Health, 11.10.2020 09:01



Spanish, 11.10.2020 09:01





Mathematics, 11.10.2020 09:01

Social Studies, 11.10.2020 09:01

English, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01

History, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01



Chemistry, 11.10.2020 09:01

German, 11.10.2020 09:01

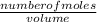
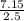 = 2.86mol/L
= 2.86mol/L

