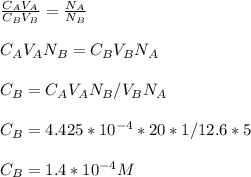Chemistry, 03.11.2020 03:20 sihamabdalla591
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O was dissolved in 200. mL of water. 20.0 mL of the solution had some acid added to it and then it reacted completely with 12.6 mL of KMnO4 solution. Calculate the concentration of the KMnO4 solution given the full REDOX equation below. 5Fe2+ + MnO4- + 8H+ --> 5Fe3+ +Mn2+ + 4H2O

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 12:30
D5w is shorthand for a 5% glucose rehydration fluid used in ivs. the doctor orders 1200 ml d5w@ 30 gtts/min. you have an iv tube that delivers 18 gtts/cc. how many hours will it take for the 1200 cc bottle to infuse?
Answers: 2

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
3.47 g of the hydrated "double salt", ammonium iron (II) sulfate hexahydrate, FeSO4(NH4)2SO4*6H2O wa...
Questions

Computers and Technology, 04.12.2019 20:31

Mathematics, 04.12.2019 20:31

English, 04.12.2019 20:31

Mathematics, 04.12.2019 20:31


Mathematics, 04.12.2019 20:31

Social Studies, 04.12.2019 20:31

History, 04.12.2019 20:31






Mathematics, 04.12.2019 20:31




Mathematics, 04.12.2019 20:31

English, 04.12.2019 20:31

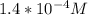
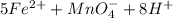 →
→ 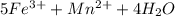
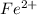 in FeSO₄(NH₄)₂SO₄*6H₂O
in FeSO₄(NH₄)₂SO₄*6H₂O 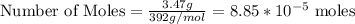
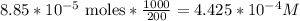
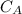 be concentration of
be concentration of 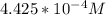
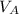 )= 20.0 ml
)= 20.0 ml
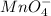 be
be 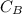 (the unknown)
(the unknown)
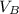 ) = 12.6 ml
) = 12.6 ml
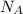 = 5 moles
= 5 moles
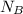 = 1 mole
= 1 mole