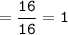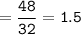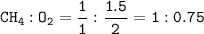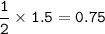Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
How many grams of carbon dioxide are produced when 16.0
g of methane and 48.0 g of oxygen gas combu...
Questions





English, 16.07.2019 18:30


History, 16.07.2019 18:30


Chemistry, 16.07.2019 18:30


Geography, 16.07.2019 18:30

Mathematics, 16.07.2019 18:30

History, 16.07.2019 18:30

English, 16.07.2019 18:30

Medicine, 16.07.2019 18:30

History, 16.07.2019 18:30



Chemistry, 16.07.2019 18:30