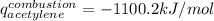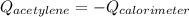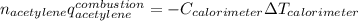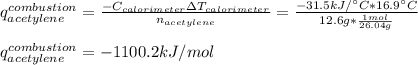Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
The heat capacity of a bomb calorimeter was determined to be 31.5 kJ/oC. A 12.6 g sample of acetylen...
Questions

English, 13.06.2020 13:57



Biology, 13.06.2020 13:57


Mathematics, 13.06.2020 13:57


Medicine, 13.06.2020 13:57


Mathematics, 13.06.2020 13:57

Biology, 13.06.2020 13:57


Physics, 13.06.2020 14:57



Social Studies, 13.06.2020 14:57