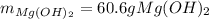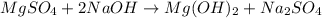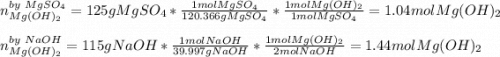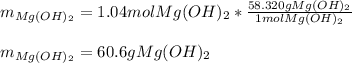Chemistry, 03.11.2020 16:40 babyleah2826
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hydroxide solution. If a solution containing 125 g of MgSO4 is added to a solution with 115 g of NaOH, what is the mass of milk of magnesia produced

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Milk of magnesia, Mg(OH)2, is prepared from the reaction of aqueous magnesium sulfate and sodium hyd...
Questions

History, 09.11.2020 20:10


Computers and Technology, 09.11.2020 20:10

Spanish, 09.11.2020 20:10




History, 09.11.2020 20:10




Mathematics, 09.11.2020 20:10

History, 09.11.2020 20:10

Mathematics, 09.11.2020 20:10

Mathematics, 09.11.2020 20:10

Mathematics, 09.11.2020 20:10



Social Studies, 09.11.2020 20:10