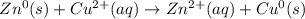Chemistry, 03.11.2020 16:50 bullockarwen
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent in the reaction of zinc with copper ions. Zn(s)+Cu2+(aq)⟶Zn2+(aq)+Cu(s) Which substance in the reaction gets reduced?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3


Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
Identify the reduced substance, the oxidized substance, the reducing agent, and the oxidizing agent...
Questions




Geography, 11.10.2019 01:10



Biology, 11.10.2019 01:10

Geography, 11.10.2019 01:10

Geography, 11.10.2019 01:10







Mathematics, 11.10.2019 01:10

Mathematics, 11.10.2019 01:10



Mathematics, 11.10.2019 01:10