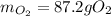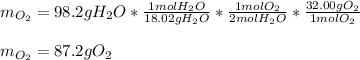Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1


Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 09:20
Which of the following occurs along coasts during the day?
Answers: 3
You know the right answer?
Gaseous hydrogen and oxygen can be prepared in the laboratory from the decomposition of gaseous wate...
Questions

Mathematics, 22.04.2020 22:29



Mathematics, 22.04.2020 22:29

Computers and Technology, 22.04.2020 22:29

Mathematics, 22.04.2020 22:29

English, 22.04.2020 22:29


Mathematics, 22.04.2020 22:29

Mathematics, 22.04.2020 22:29

History, 22.04.2020 22:29

History, 22.04.2020 22:29


History, 22.04.2020 22:29

Mathematics, 22.04.2020 22:29

Mathematics, 22.04.2020 22:29