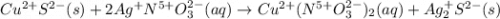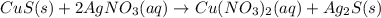Chemistry, 03.11.2020 16:50 ReonRamseyz
g Solid copper sulfide and silver nitrate react to form copper (II) nitrate and solid silver sulfide. Write a balanced chemical equation that describes the reaction. Identify the oxidation number of each element in the reaction as well as the physical state of each compound. You do not need to include the total contribution of charge. Is this reaction a redox reaction or a non-redox reaction

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
g Solid copper sulfide and silver nitrate react to form copper (II) nitrate and solid silver sulfide...
Questions

Mathematics, 04.05.2021 20:20


English, 04.05.2021 20:20

Biology, 04.05.2021 20:20




Mathematics, 04.05.2021 20:20



Mathematics, 04.05.2021 20:20


English, 04.05.2021 20:20

Business, 04.05.2021 20:20




English, 04.05.2021 20:20

Mathematics, 04.05.2021 20:20