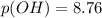A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The of aniline is . Round your answer to decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of solution added.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
A chemist titrates of a aniline solution with HCl solution at . Calculate the pH at equivalence. The...
Questions










French, 22.10.2020 16:01





Computers and Technology, 22.10.2020 16:01






 = 0.044 M
= 0.044 M![p(OH)=\frac{1}{2} [pKw+pKb+logC]](/tpl/images/0863/4823/b2809.png)
![p(OH)=\frac{1}{2} [14+4.87+log0.044]](/tpl/images/0863/4823/70b78.png)