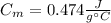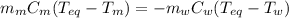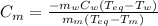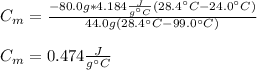Chemistry, 03.11.2020 17:20 Justadumbemo
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negligible heat capacity containing 80.0 mL water at 24.0 oC. The final temperature of the system was found to be 28.4 oC. Calculate the Specific heat of the metal if density of water is 1.00 g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


You know the right answer?
A 44.0 g sample of an unknown metal at 99.0 oC was placed in a constant-pressure calorimeter of negl...
Questions



History, 29.07.2019 19:00


Biology, 29.07.2019 19:00



History, 29.07.2019 19:00


Mathematics, 29.07.2019 19:00


Mathematics, 29.07.2019 19:00

Chemistry, 29.07.2019 19:00

Business, 29.07.2019 19:00



Mathematics, 29.07.2019 19:00