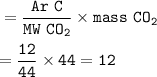Chemistry, 04.11.2020 14:00 kendramiller3965
BASED ON THE INFORMATION BELOW. CALCULATE THE MASS FOR ONE OF THESE ATOMS:
MASS OF A SINGLE CARBON ATOM:
MASS OF A SINGLE OXYGEN ATOM:
MASS OF A SINGLE HYDROGEN ATOM:


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2
You know the right answer?
BASED ON THE INFORMATION BELOW. CALCULATE THE MASS FOR ONE OF THESE ATOMS:
MASS OF A SINGLE CARBON...
Questions


Biology, 07.11.2019 03:31



Advanced Placement (AP), 07.11.2019 03:31

Mathematics, 07.11.2019 03:31


Advanced Placement (AP), 07.11.2019 03:31





History, 07.11.2019 03:31

Physics, 07.11.2019 03:31

Physics, 07.11.2019 03:31

Social Studies, 07.11.2019 03:31


Mathematics, 07.11.2019 03:31


Biology, 07.11.2019 04:31