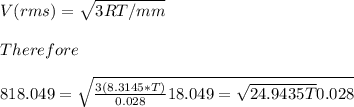Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3
You know the right answer?
Calculate the temperature (K) of N2 gas if the molecules have a speed of 818.049 m/s...
Questions

Mathematics, 26.02.2021 04:00

English, 26.02.2021 04:00


Mathematics, 26.02.2021 04:00

SAT, 26.02.2021 04:00


History, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00

History, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00



Mathematics, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00

Spanish, 26.02.2021 04:00

Mathematics, 26.02.2021 04:00